Efficacy of niraparib by timing of surgery and residual disease: a post-hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study - Gynecologic Oncology
Zejula (Niraparib) First PARP Inhibitor Approved for First-Line Maintenance Therapy in All Women with Advanced Ovarian Cancer, Regardless of Biomarker Status

Real-world adverse events with niraparib 200 mg/day maintenance therapy in ovarian cancer: a retrospective study | Future Oncology

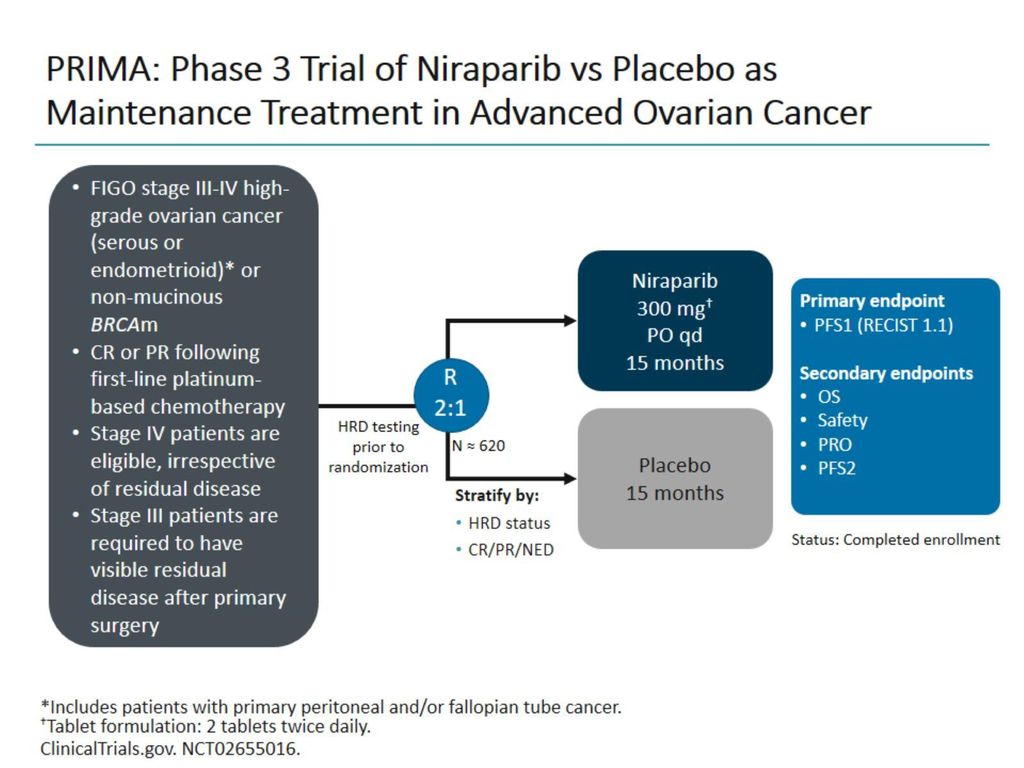
Prospective evaluation of the tolerability and efficacy of niraparib dosing based on baseline body weight and platelet count : results from the PRIMA/ENGOT-OV26/GOG-3012 trial

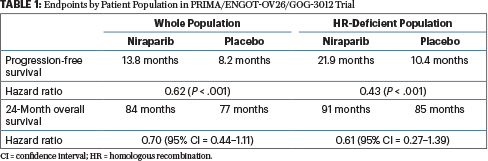
Progression-free survival and safety at 3.5 years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer - European Journal of

Progression-free survival and safety at 3.5 years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer - ScienceDirect

Stephen V Liu, MD on X: "#ESMO19 Practice changing study presented by Antonio Gonzalez Martin. PRIMA study of niraparib in patients with ovarian cancer responding to 1L platinum based chemo. Primary endpoint

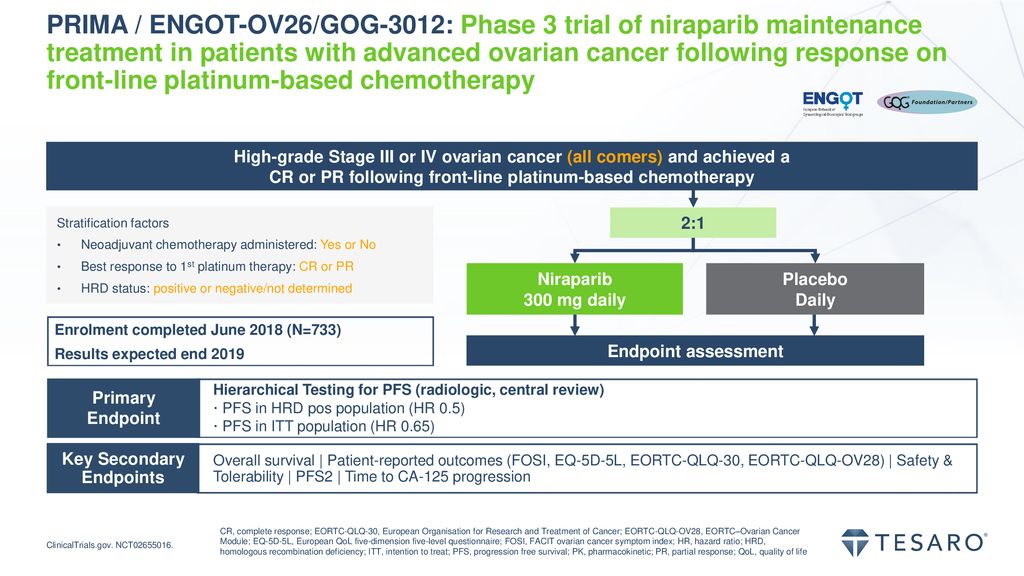
Efficacy and safety of niraparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer using an individualized starting dose (PRIME Study): A randomized, double-blind, placebo-controlled, phase 3 trial (LBA 5) -
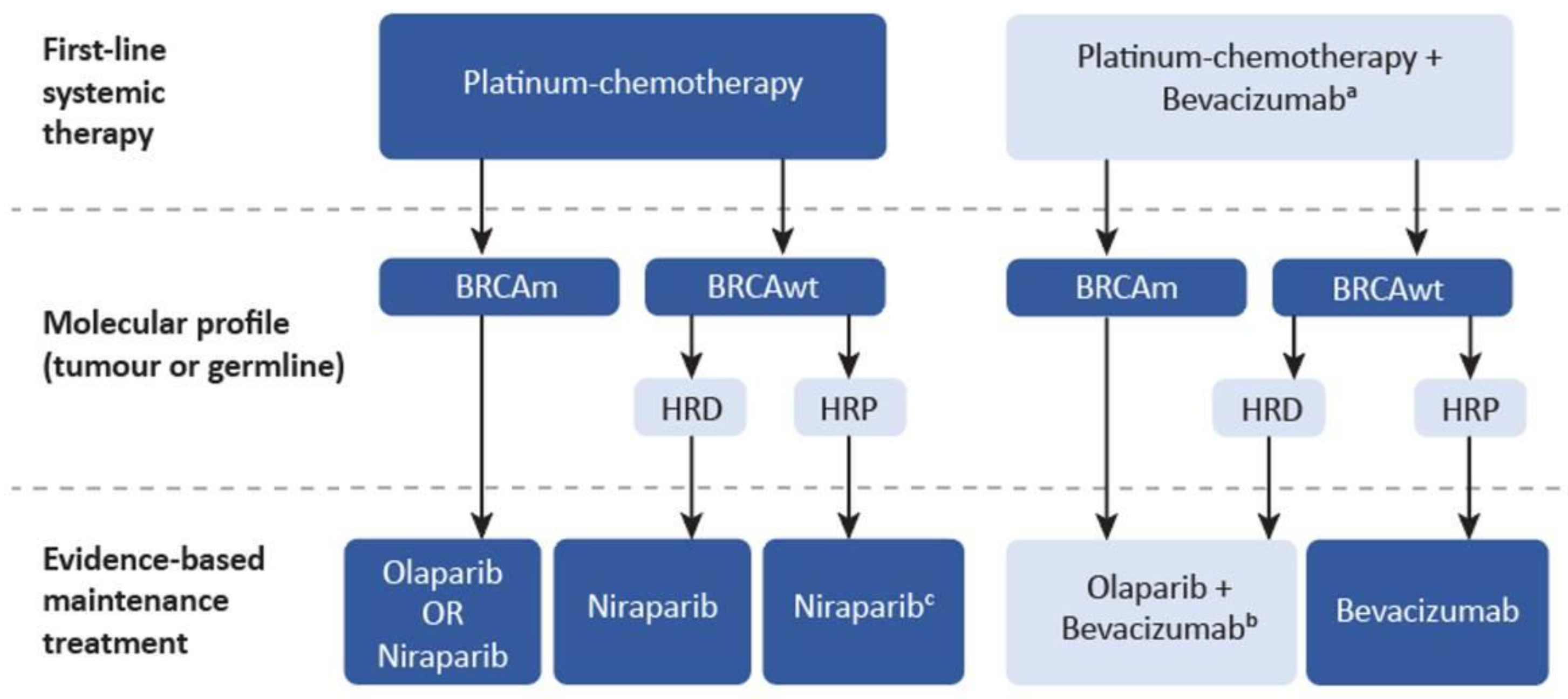
Current Oncology | Free Full-Text | A Pan-Canadian Consensus Statement on First-Line PARP Inhibitor Maintenance for Advanced, High-Grade Serous and Endometrioid Tubal, Ovarian, and Primary Peritoneal Cancers

Quality-adjusted time without symptoms of disease or toxicity and quality-adjusted progression-free survival with niraparib maintenance in first-line ovarian cancer in the PRIMA trial
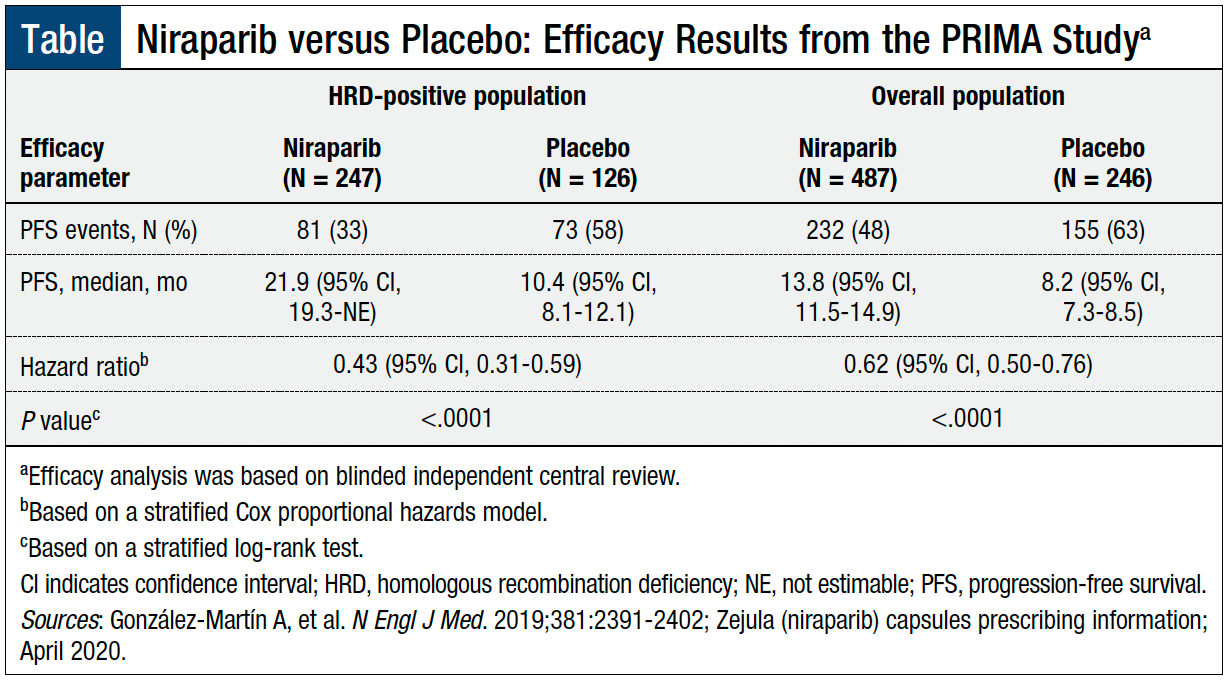
Niraparib Efficacy and Safety in Patients with BRCA-Mutated (BRCAm) Ovarian Cancer: Results from Three Phase 3 Niraparib Trials